As of Spring 2023, MSK now has an institutional subscription to Mendeley Reference Manager. Mendeley Reference Manager is a “web-based citation manager that helps you simplify the tasks of building and organizing your reference library, making notes and annotations across papers, collaborating with others, and inserting citations and bibliographies into the papers you’re writing.”
How do I use my MSK institutional credentials with Mendeley?
Creating a new Mendeley account connected with your MSK institutional credentials
Connect an existing Mendeley or Elsevier account to your MSK institutional credentials
Like traditional citation management tools (for example, EndNote), Mendeley allows users to easily harvest and manage their references, to read and annotate their PDF attachments, and to cite research and format their bibliographies while they write. Similar to other social reference managers like Zotero, however, Mendeley also has some online collaboration features and academic social networking functionality that are definitely worth exploring.
Most notably, Mendeley lets users share their references and annotated PDFs using groups.
With MSK’s institutional subscription to Mendeley, users can now take advantage of 100 GB of personal storage (versus 2 GB in the free version), 100 GB of team storage (versus 2 GB in the free version), an unlimited number of private groups (versus 5 in the free version), and the ability to have 100 members per group (versus 25 in the free version).
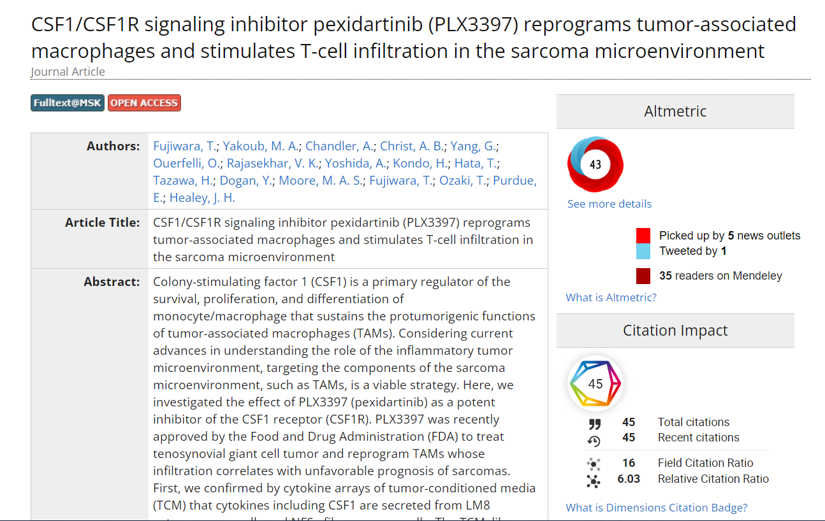
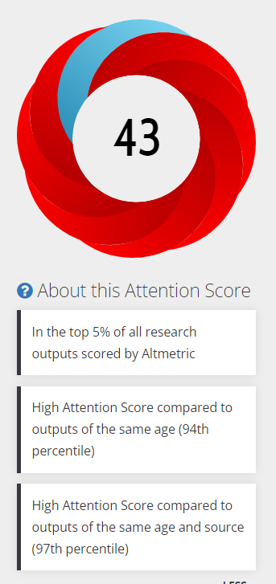
Launched in 2008 but acquired by Elsevier in 2013, Mendeley is well-integrated into tools like Scopus and ScienceDirect that makes logging in and exporting citations to seamless. Beyond helping with citation management and formatting bibliographies, the Mendeley platform collects data on how users interact with scholarly documents, generating anonymized usage data about its readers that it then openly-shares (via the Mendeley API) with tools like Altmetrics, where the data serves as one type of social media attention or alternative metric of research impact (see an example in the recent MSK Library blog post “View the Impact of Your Research in Synapse”.
To learn more about Mendeley, explore the Mendeley training guides available on the vendor’s website and/or view a quick product video overview (2:24 min). In the upcoming weeks, please do keep an eye on the MSK Library’s Citation Management LibGuide and the training class calendar for much more to come on Mendeley.
Further reading:
Elston DM. Mendeley. J Am Acad Dermatol. 2019 Nov;81(5):1071. doi: 10.1016/j.jaad.2019.06.1291. Epub 2019 Jul 3. PMID: 31279032.
Chen PY, Hayes E, Larivière V, Sugimoto CR. Social reference managers and their users: A survey of demographics and ideologies. PLoS One. 2018 Jul 11;13(7):e0198033. doi: 10.1371/journal.pone.0198033. Erratum in: PLoS One. 2018 Aug 9;13(8):e0202315. PMID: 29995889; PMCID: PMC6040870.
Thelwall, M. (2018). Early Mendeley readers correlate with later citation counts. Scientometrics, 115(3), 1231-1240.
Questions? Ask Us at the MSK Library!