Through its sponsorship of its Evidence-Based Practice Centers (EPCs), the Agency for Healthcare Research Quality (AHRQ) provides a searchable database of Evidence-Base Reports on its website. These reports inform the audience on medical conditions, new healthcare technologies, and strategies through a comprehensive evidence-based approach.
Some of the database features include: filtering by topic, date, document type and organization or institution, as well as setting up email alerts for updates.
To search the database:
* Click: EBC Evidence-Based Reports
* Click on SEARCH ALL EPC REPORTS
* Enter a keyword in the search box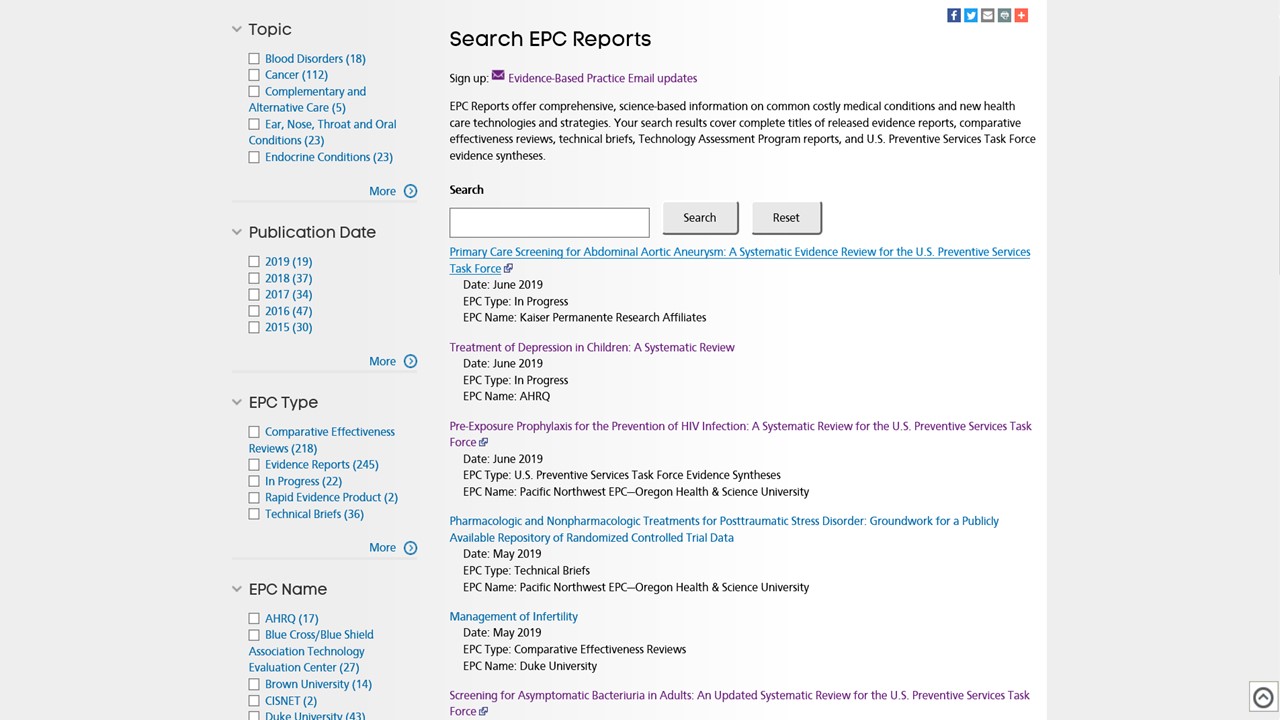
Link to the AHRQ website from the MSK Library’s Databases, then hover over Research and click on EPC Evidence-Base Reports. If you have any questions about this resource, please don’t hesitate to ASK US.
