The MSK Library supports researchers working on systematic review (SR) projects with a variety of resources and services and training opportunities. An additional SR tool option available to MSK users, that is included as part of the library’s subscription to the Joanna Briggs Institute (JBI) EBP Database (OVID), is an “EBP Tool” called JBI SUMARI.
According to the vendor:
This comprehensive software suite has been developed to assist users on developing, conducting and reporting on systematic reviews of evidence related to the feasibility, appropriateness, meaningfulness and effectiveness of health care interventions or professional activities.
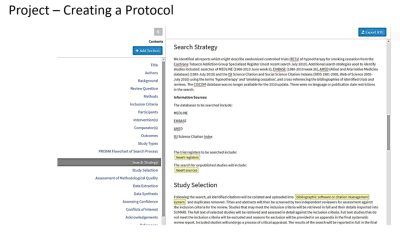
Similar to Covidence (another systematic review project management software tool currently available to MSK researchers), JBI SUMARI includes functionality for: Project Management, Protocol Builder, Import of Studies, Study Screening, Assessment of Risk of Bias, Data Extraction, Data Synthesis, and Report Writing. To learn more about what you can do with JBI SUMARI and how it can be used in conjunction with Covidence (to make the most of certain functionality that may be missing in one or the other of these tools), be sure to read this published review:
Piper C. System for the Unified Management, Assessment, and Review of Information (SUMARI). J Med Libr Assoc. 2019 Oct;107(4):634–6. doi: 10.5195/jmla.2019.790. Epub 2019 Oct 1. PMCID: PMC6774554.
The JBI SUMARI Knowledge Base is also a great place to look for guidance on using this tool. Two areas worth noting where JBI SUMARI differentiates itself from Covidence are with its built-in Protocol Builder capabilities and its Synthesis functionality – features that are currently not available in Covidence.
Questions? Ask Us at the MSK Library!