A frequent question we are asked by our users is how to retrieve only research (or peer-reviewed) articles from Synapse. Previously our “Journal Articles” category included other formats such as editorials, commentaries, letters, reviews, guidelines, interviews etc. Identifying just research articles was difficult without looking through the Synapse records one by one – but no longer! Beginning with 2020 publications and moving forward, we have refined this category to separate the various formats, allowing our users to more precisely retrieve their desired publication data. We will continue to also filter meeting abstracts, conference papers, book chapters, and whole books.
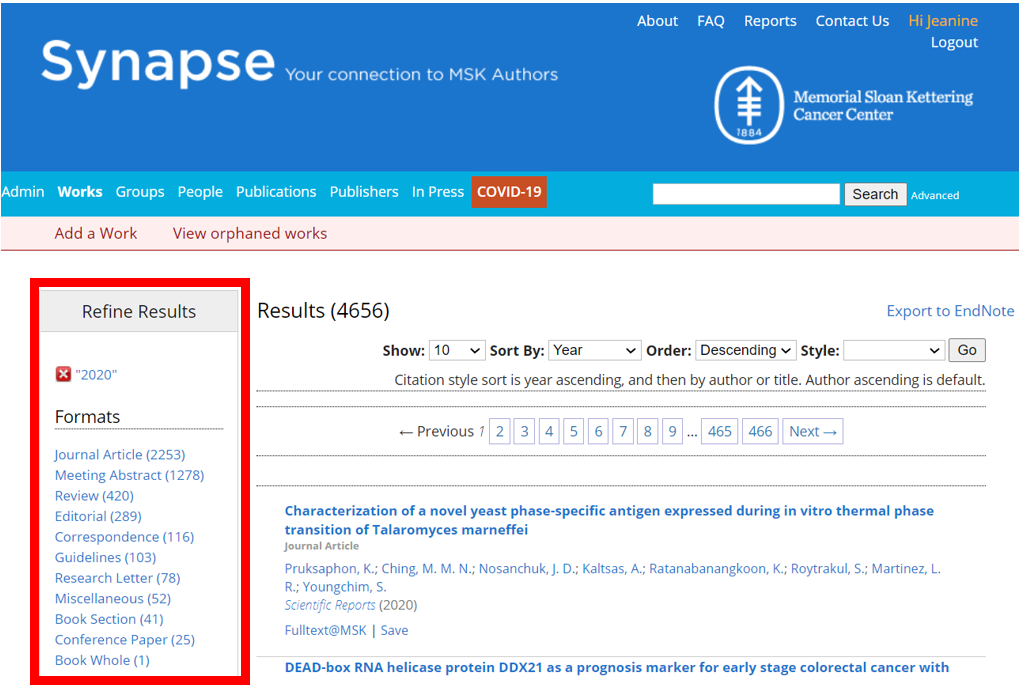
Here are our new categories (see full list):
Correspondence: letters to the editor, replies, and other items included in the correspondence sections of journals.
Editorial: editorials, viewpoints, commentaries, and other opinion type pieces published in journals. Also includes sections of a journal typically authored by the editorial board, such as the preface or introduction to a special issue.
Guidelines: a set of guidelines/recommendations written on behalf of a professional group or society. May also be called a consensus statement. Examples include guidelines and related updates provided by the National Comprehensive Cancer Network.
Journal Article: presentation of original research investigations that are likely, but not always, peer-reviewed. This category includes studies such as: clinical trials, case reports, brief reports, meta-analyses, laboratory investigations, retrospective analyses, CME activities, and white papers.
Miscellaneous: items that do not fall within other categories such as: book reviews, interviews, obituaries, debates, podcasts etc. These works are usually not peer-reviewed.
Research Letter: original research presented in journals as correspondence.
Review: comprehensive literature, topic, or subject summaries, including systematic reviews.
If you have any questions, please don’t hesitate to contact us.
Happy New Year!