An article in Futurity details the findings of a paper in the Journal of Immunological Methods on a unique, new test that could one day detect diseases like cancer. The technique generated a massive library of random molecular shapes to can detect antibodies in blood, and may one day lead to new screening tests for a variety of non-microbial causing diseases.
Category Archives: Do You Know?
The FDA is Seeking to Regulate 3D Printing for Medical Devices
3D printing of medical devices is becoming more commonplace in biomedical sciences. There has not been any official set of guidelines from the U.S. Food and Drug Administration (FDA), however. As a result of this increase in 3D printing, the FDA just issued a draft guidance for 3D-printed medical devices (or additive manufactured [AM] devices). The FDA has so far approved nearly 100 applications for lower-risk 3D printed medical devices, but the organization is seeking to make the application process more efficient. How will this affect physicians who wish to 3D-print a medical device in his or her own workplace?

The draft guidance stems from a 2014 public workshop and was created to help medical device manufacturers follow the FDA’s views on 3D printing. The FDA’s guidance for manufacturers “outline[s] technical considerations associated with AM processes, and recommendations for testing and characterization for devices that include at least one AM fabrication step.” It outlines how manufacturers need to think about device design, the effects of imaging (MRIs, CT scans, etc.), software workflow, material controls, post-processing validation of the biomedical devices, quality control and testing, and cleaning and sterilization procedures.
Continue reading
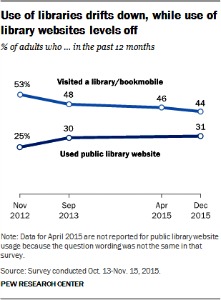
Do You Know the Key Services Your Library Provides?
The Pew Research Center recently released a report on Americans’ thoughts on how well they believe their local libraries serve their users and how library users excel over others in learning activities. Interestingly, these same users are not all aware of important educational services their libraries provide. This begs the question, are you aware of the key services the Memorial Sloan Kettering Cancer Center Medical Library provides to you?