- Stress can re-ignite dormant tumor cells to cause cancer recurrence months or years after completing of successful treatment. A mixed animal and human study by an international group of researchers established that norepinephrine and cortisol, stress hormones released into the bloodstream when the level of stress is elevated, start a chain of biochemical events ultimately leading to reactivating the tumor cells. One of the findings in the human part of the study was the beta-blocker’ class of drugs ability to inhibit stress hormone signaling which prevented the reactivation of cancer cells. This finding opens up the possibility of beta-blockers use for cancer recurrence prevention. The study was published in Science Translational Medicine.
- Harvard’s Wyss Institute for Biologically Inspired Engineering and John A. Paulson School for Engineering and Applied Sciences (SEAS) used an unconventional approach to managing hard to treat lung metastases. They delivered immune system stimulating chemicals directly into the lung metastasis via drug-filled nanoparticles attached to red blood cells thus sparing the healthy lung tissues otherwise damaged by chemotherapy. The scientists also established that this method could halt further lung cancer growth and also prevent cancer recurrence. This animal research paves the way to new therapeutic options for metastatic cancers. The study was published in Nature Biomedical Engineering.
- Another team from Harvard’s Wyss Institute for Biologically Inspired Engineering and John A. Paulson School for Engineering and Applied Sciences (SEAS) developed a vaccine that combines chemo and immunotherapy in one injection. This animal research shows some promise even in difficult to treat cancers. The study was published in Nature Communications.
- Researchers at the Centre for Genomic Regulation (CRG) in Barcelona and Columbia University in New York City found a way to produce more hematopoietic stem cells (HSCs) – self-renewing stem cells crucial for treating cancer, as well as other serious diseases. The scarcity of such cells has always presented a problem. While these cells are typically derived from bone marrow and circulating and cord blood this research established another way of getting them – by reprogramming other blood stem cells. The researchers used a special algorithm to identify a gene capable of reprogramming blood stem cells to acquire hematopoietic stem cell properties. This research boosts the opportunity for more patients to benefit from hematopoietic stem cell treatments. The study was published in Cell Reports.
Category Archives: Cancer Research News
Delaying Cancer Treatments and Impact on Mortality
In the unprecedented time of COVID-19, there is a dire need to understand the impact of delaying cancer treatment. A team from Canada and the United-Kingdom undertook a Systematic Review and Meta-Analysis of studies published from January 2000 to April 2020 to quantify the impact of delays in surgical interventions, systemic treatment (such as chemotherapy), or radiotherapy for seven types of cancer. These seven cancers represent “44% of all incident cancers globally”.
From analyzing studies included in the review, the authors concluded that every four-week delay in cancer treatment was associated with increased mortality. Delaying more than four weeks was associated with an even higher increase in the risk of death. For instance, delaying breast cancer surgery for eight weeks would increase the risk of death by 17%. This risk would be 26% with a twelve-week delay.
The study was published in November 2020 in the BMJ. The findings of this Systematic Review and Meta-Analysis will be instrumental in creating policies on cancer management priorities during a pandemic.
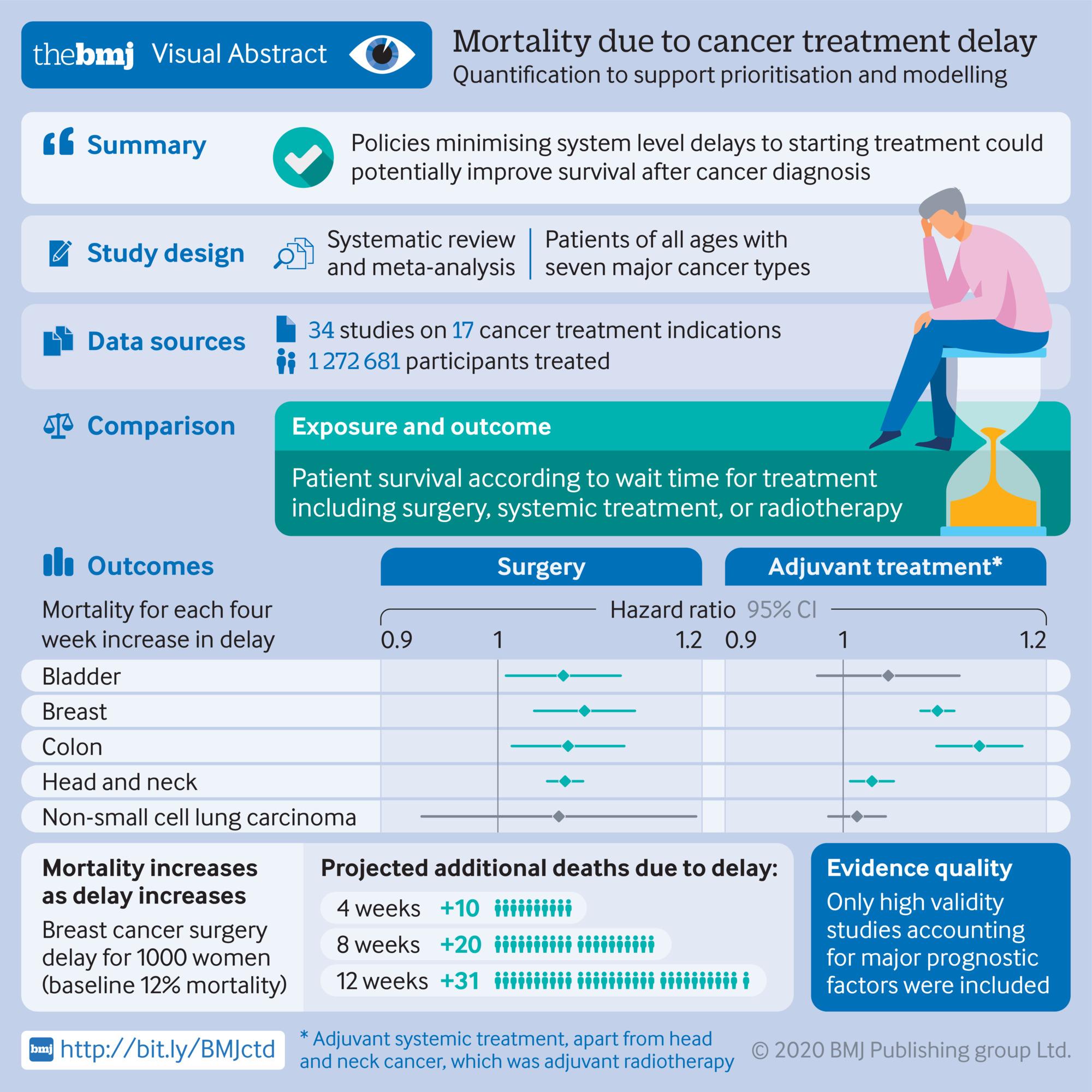
Hanna, T. P., King, W. D., Thibodeau, S., Jalink, M., Paulin, G. A., Harvey-Jones, E., O’Sullivan, D. E., Booth, C. M., Sullivan, R., & Aggarwal, A. (2020). Mortality due to cancer treatment delay: systematic review and meta-analysis. BMJ (Clinical research ed.), 371, m4087. https://doi.org/10.1136/bmj.m4087
Overcoming Resistance to Cancer Therapies, Nanomedicine and More
Resistance to cancer therapy is one of the most challenging problems in oncology. Two recent papers report on new findings related to the phenomena of such resistance.
- A UCSF team of researchers found that the presence of liver metastasis from any primary cancer causes resistance to immune checkpoint inhibitors therapy, specifically to anti–PD-1 immunotherapy. Using a mouse model, the scientists established a foundation for overcoming this resistance by combining anti-PD-1 with regulatory T cells targeting agents and thus restoring anti-PD-1 immunotherapy efficacy. The study was published in Science Immunology.
- BRCA1/2 mutation-driven cancers, such as breast, ovarian, and prostate cancers, display high resistance to lifesaving therapies. Researchers from The University of Texas at Austin and Ajou University in South Korea identified a protein implicated in developing resistance to PARP inhibitors, a class of drugs that treat BRCA-deficient tumors. The study found that the low level of this protein, called PCAF, causes resistance to treatment with PARP inhibitors. The findings open the possibility of overcoming this resistance and increasing PARP inhibitors therapy’s effectiveness by combining them with a class of drugs called HDAC inhibitors, which boost PCAF. Research into PCAF protein, which has a role in chromatin modifications responsible for important DNA processes, contributes to the knowledge of cell replication and, therefore, to the understanding of disease pathophysiology. The study was published in Molecular Cell.
In recent years, anti-cancer nanomedicine gained more and more ground.
- Researchers from two laboratories in Chicago conducted a recent study on nanotechnology that used charged nanoscale metal-organic frameworks (nMOFs) “for generating free radicals using X-rays within tumor tissue to kill cancer cells directly”. “Furthermore, the same frameworks can be used for delivering immune signaling molecules known as PAMPs to activate the immune response against tumor cells. By combining these two approaches into one easily administered “vaccine,” this new technology may provide the key to better local and systemic treatment of difficult-to-treat cancers”. This study was published in Science Advances.
- Yet another study on nanotechnology took a non-conventional approach to nanoparticle use. A research team from Singapore used a silica nanoparticle as a cancer drug, instead of a conventional drug carrier. The therapeutic nanoparticle caused cancer cells to self-destruct with the same efficiency as traditional cancer drug therapy in the lab mouse experiment. The researchers also “deceived” cancer cells, notoriously dependent on amino acids for their growth, by masking the therapeutic nanoparticle with an outer layer of amino acid L-phenylalanine. This research “may hold promise for the future design of nanotherapies” and “for cancer cells that have failed to respond to conventional treatment like chemotherapy.” The study was published in Small.
More studies contributing to the knowledge of cancer biology were published recently.
- Scientists from Rockefeller University in New York found that breast and lung tumor cancer cells can use blood vessels to gain access to a signaling pathway used by neurons. The tactics ultimately enable those cancers to metastasize. This research contributes to the knowledge of how cancers use or hijack normal cells and mechanisms to progress and establishes the foundation for new diagnostic and therapeutic approaches. The study was published in Nature.