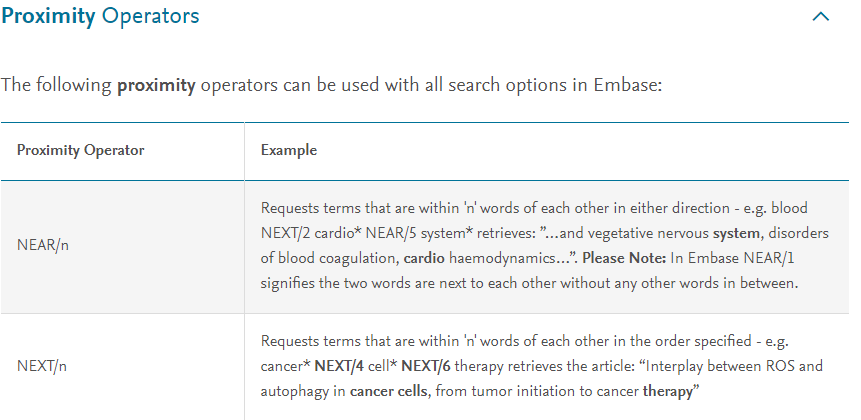
The latest version of PubMed has made a significant change behind the scenes that for more sophisticated PubMed users, needs to be addressed. If a PubMed user manually types in a subject heading, a MeSH term, in the search box, double quotation marks should now be used around these terms even with the [MeSH], [MH], or [Majr] fields; otherwise PubMed will automatically map to a variety of Mesh terms.
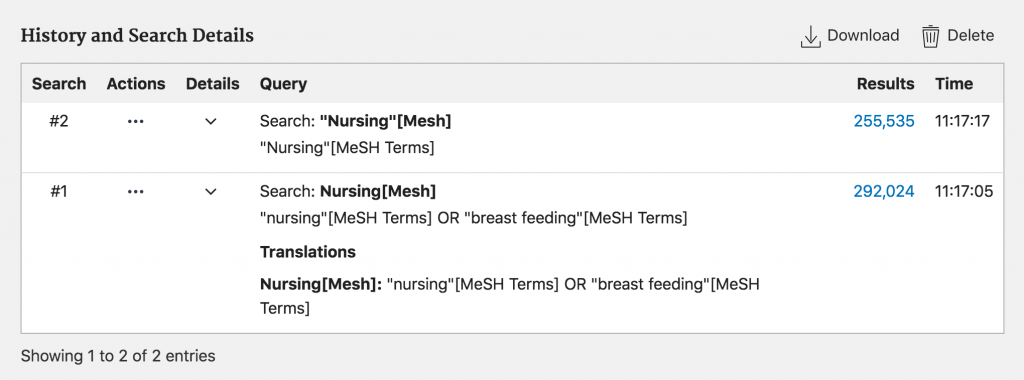
There can be times when PubMed’s mapping of [Mesh] designated terms can be useful. If, for instance, you knew that the Mesh term for cancer was neoplasm, but did not make it plural, using double quotation marks would retrieve no results while not using quotation marks would retrieve relevant results as PubMed is able to map to the correct Mesh term.
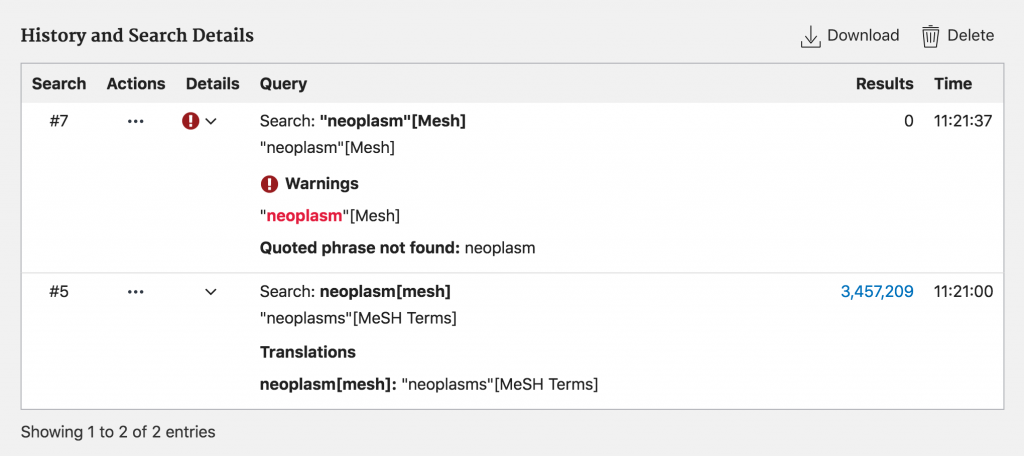
Even though manually typing in Mesh terms into the search bar can be more efficient, it can also become problematic. It is advised to simply use the MeSH Database to not only ensure that you are inputting the terms properly, but also to ensure that you are using the correct term for the concept you are searching for!