Searching efficiently for clinical trials is often challenging for researchers. The majority of clinical trials are not published in peer-review publications. Therefore, the results are never reported and disseminated (1). Additionally, there are multiple clinical trial registries with considerable overlap (2).
While the aim of the International Clinical Trials Registry Platform (ICTRP), launched in 2007 by the World Health Organisation (WHO) (3), is to act as a single platform to search for clinical trials, it has been reported by Glanville and Knelangen that “even though ClinicalTrials.gov is included in the WHO ICTRP Search Portal, not all ClinicalTrials.gov records can be successfully retrieved via searches of the ICTRP Search Portal”(4). To be comprehensive when searching for clinical trials, we will need to search across multiple registers.
Here are some steps that may be helpful when searching for clinical trials:
Finding clinical trials from registries:
- International Clinical Trials Registry Platform (ICTRP)
The ICTRP is maintained the World Health Organization.
Please note that as of January 2021, the ICTRP search portal is experiencing a high volume search inquiries because of COVID-19 and may not be responsive. - ClinicalTrials.gov
ClinicalTrials.gov is provided by the National Library of Medicine (NLM). Studies registered on the website are from all 50 United States and 219 countries. - The European Union (EU) Clinical Trials Register
The register contains studies conducted in the European Union (EU) and the European Economic Area (EEA) as well as studies conducted outside the EU / EEA that are linked to European paediatric-medicine development. - The following website is a comprehensive resource with links to national and international clinical trials registers: https://sites.google.com/a/york.ac.uk/yhectrialsregisters/home/clinicaltrials
Finding clinical trials with published results:
- PubMed clinical queries
A broad therapy search will retrieve clinical trials, if you are interested in Randomized Clinical trials only, you can change the scope from broad to specific/narrow.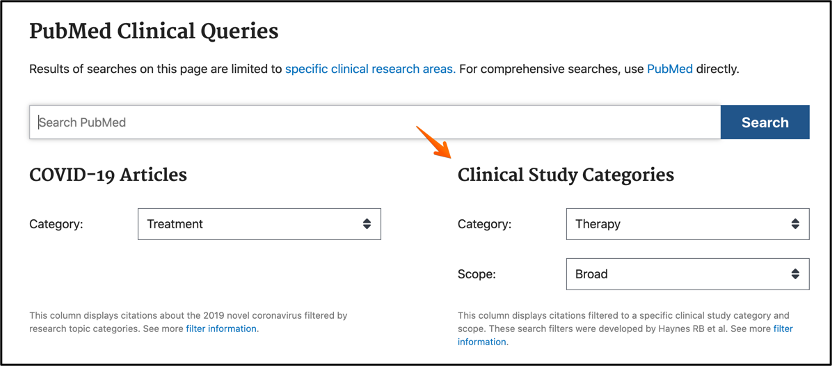
- The Cochrane Central Register of Controlled Trials (CENTRAL)
The records are pulled mainly from the databases PubMed and Embase.
-
ClinicalTrials.gov
In the advanced search, you can select “Studies with Results” to retrieve only studies where the results have been reported: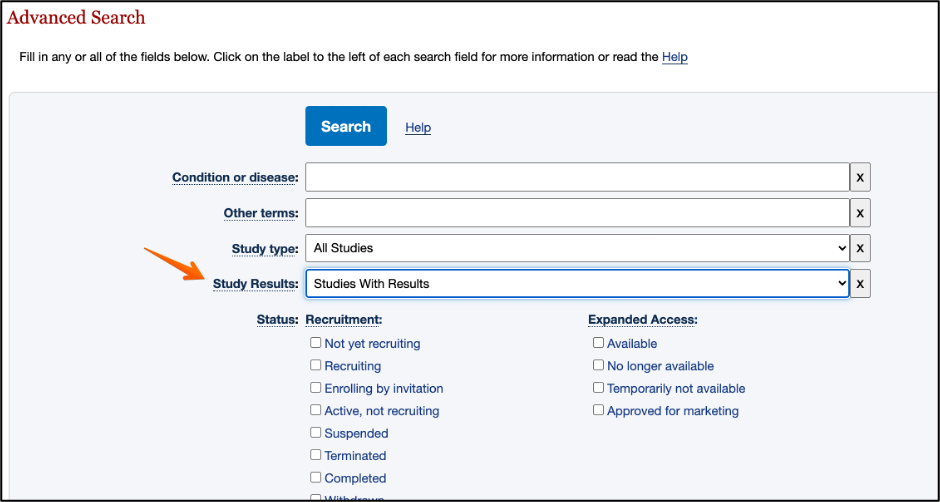
Finding clinical trials for COVID-19:
- Trialstreamer
“Trialstreamer is an artificial intelligence system, which finds and summarises new trial publications, registrations, and preprints in COVID-19.” - Global Coronavirus COVID-19 Clinical Trial Tracker
A real-time dashboard of clinical trials for COVID-19 developed by Thorlund, Kristian, et al. - Anticovid platform
“Anticovid is an open access platform which gathers all available information regarding global clinical trials for SARS-CoV 2.”
For information on clinical trials at MSKCC, please visit the following page: https://www.mskcc.org/cancer-care/clinical-trials
References
- DeVito NJ, Bacon S and Goldacre B. Compliance with legal requirement to report clinical trial results on ClinicalTrials.gov: a cohort study. The Lancet 2020; 395: 361-369. DOI: 10.1016/S0140-6736(19)33220-9.
- van Valkenhoef G, Loane RF and Zarin DA. Previously unidentified duplicate registrations of clinical trials: an exploratory analysis of registry data worldwide. Systematic Reviews 2016; 5: 116. DOI: 10.1186/s13643-016-0283-8.
- Karam G and Ross AL. The WHO International Clinical Trials Registry Platform: Providing global clinical trial information to all. On Medicine. 2020.
- Lefebvre C GJ, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, Noel-Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. . Chapter 4: Searching for and selecting studies. In: Higgins JPT TJ, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (ed) Cochrane Handbook for Systematic Reviews of Interventions version 61 (updated September 2020). Cochrane, 2020.