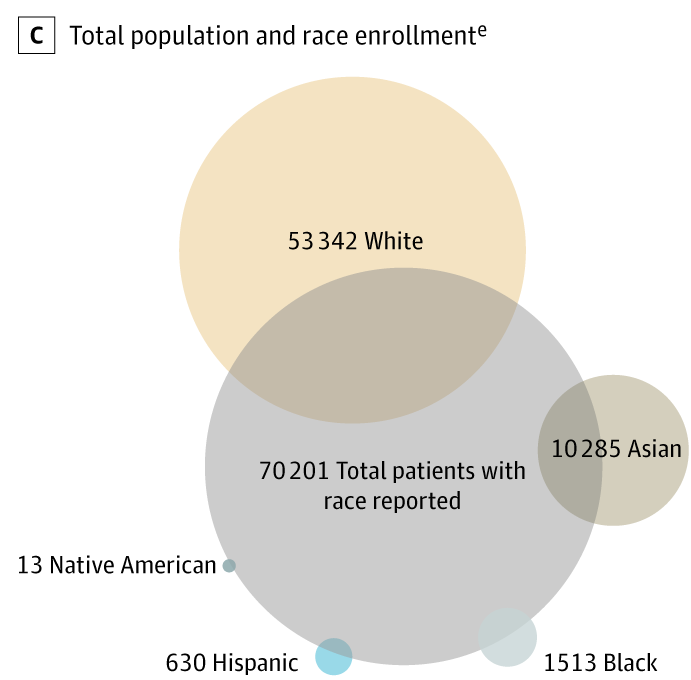
Figure 1, Part C: The absolute number of patients of races other than white who participated in pivotal trials leading to FDA approval was considerably low. From: Loree JM, Anand S, Dasari A, et al. Disparity of Race Reporting and Representation in Clinical Trials Leading to Cancer Drug Approvals From 2008 to 2018. JAMA Oncol. Published online August 15, 2019. doi:10.1001/jamaoncol.2019.1870
Back in 2004, a study published in JAMA indicated that racial and ethnic minorities, women, and the elderly were less likely to enroll in cooperative group cancer clinical trials than were whites, men, and younger patients, respectively. A more recent ProPublica examination showed that black people and Native Americans are under-represented in clinical trials of new drugs, even when the treatment is aimed at a type of cancer that disproportionately affects them.
Dr. Mona Fouad suggests that one possible strategy to increase minority participation in clinical trials may be patient navigators. A 2016 study she co-authored on patient navigation demonstrated that the patient navigation model “holds promise as a strategy to reduce disparities in cancer clinical trial participation” and that “future studies should evaluate it with racial/ethnic minorities across cancer centers.”